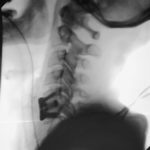
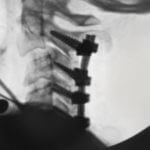
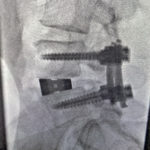
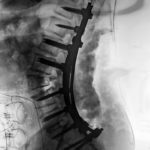
PTP™ is NOT Prone Lateral
Learn how PTP utilizes a maximally efficient prone position while providing a consistently reproducible approach, enabling surgeons to achieve powerful alignment.
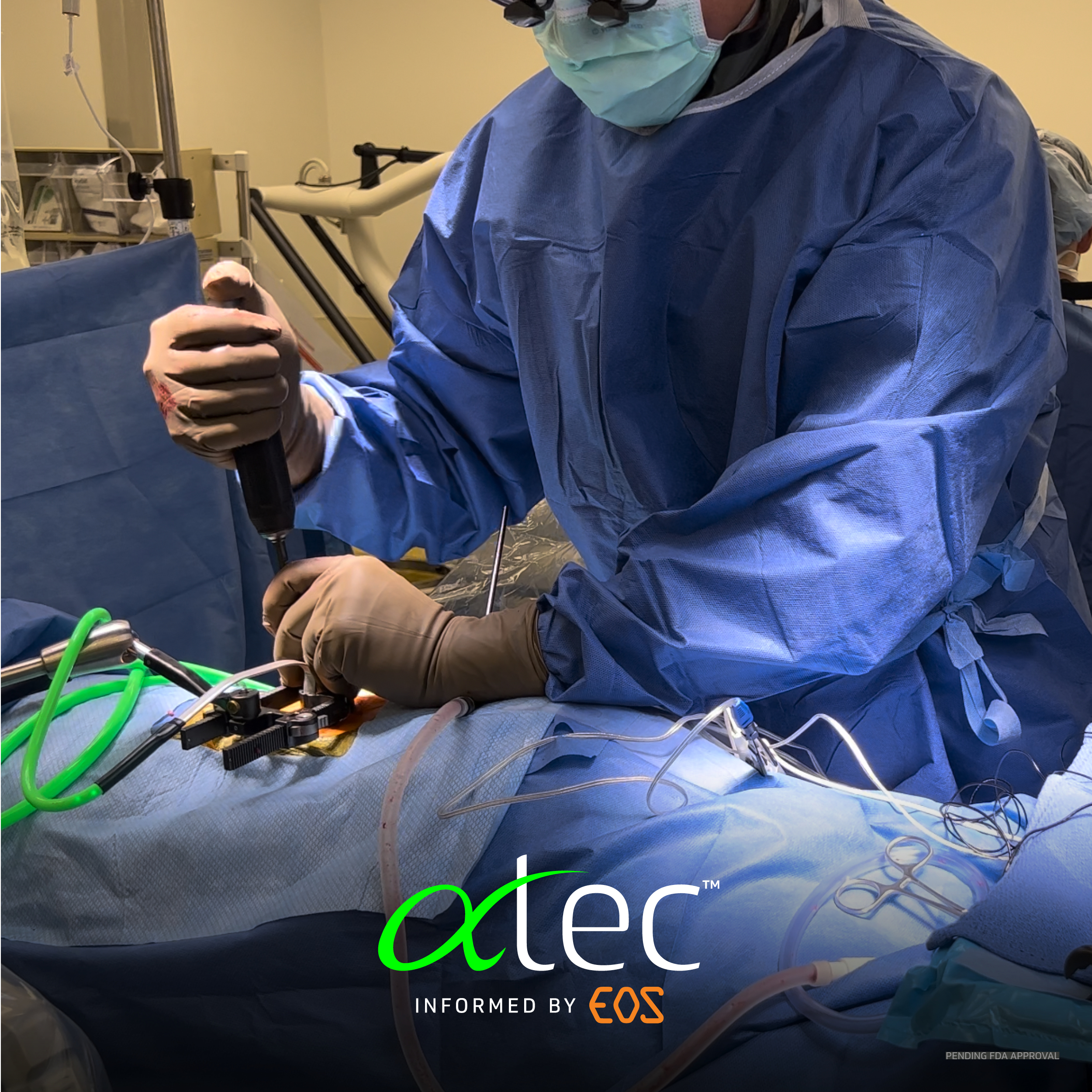
Informing Better Surgery
Hear why an ATEC | EOS-informed surgery will elevate the likelihood of achieving the surgeon’s alignment objectives - and there is no greater predictor of successful, long-term clinical outcomes than spinal alignment.
Latest News
ATEC Reports Fourth Quarter and Full-Year 2023 Financial Results
• Full year 2023 total revenue grew 37% to $482 million • Full year 2023 adjusted EBITDA margin improved ~890 basis points • Full year 2024 total revenue expected to […]
VIEW PRESS RELEASEATEC Equipped to Support Ramping Surgical Volume With Proceeds From Secondary Offering
Positioned to invest in revenue-generating assets as Company continues to capitalize on industry disruptions CARLSBAD, Calif., October 31, 2023 – Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions […]
VIEW PRESS RELEASEATEC Clinical Precision and Predictability on Display at NASS 2023
Unleashing 100% spine focus to set the standard in spine care CARLSBAD, Calif., October 9, 2023 – Alphatec Holdings, Inc. (Nasdaq: ATEC), a provider of innovative solutions dedicated to revolutionizing […]
VIEW PRESS RELEASE